Highlights
•
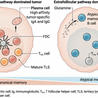
Innate immune cells, specifically NK cells and macrophages, play an important role in tumor clearance by antitumor antibodies.
•
Co-stimulatory and checkpoint blockade antibodies can augment the effector functions of innate immune cells.
•
The combination of antibodies targeting innate effectors with tumor-targeted antibodies offers a promising new paradigm for cancer therapy.
Cancer immunotherapy is a rapidly evolving field that offers a novel paradigm for cancer treatment: therapies focus on enhancing the immune system's innate and adaptive anti-tumor response. Early immunotherapeutics have achieved impressive clinical outcomes and monoclonal antibodies are now integral to therapeutic strategies in a variety of cancers. However, only recently have antibodies targeting innate immune cells entered clinical development. Innate immune effector cells play important roles in generating and maintaining antitumor immunity. Antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) are important innate immune mechanisms for tumor eradication. These cytolytic processes are initiated by the detection of a tumor-targeting antibody and can be augmented by activating co-stimulatory pathways or blocking inhibitory signals on innate immune cells. The combination of FDA-approved monoclonal antibodies with innate effector-targeting antibodies has demonstrated potent preclinical therapeutic synergy and early-phase combinatorial clinical trials are ongoing.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...


![The IL-20 subfamily of cytokines [mdash] from host defence to tissue homeostasis : Nature Reviews Immunology : Nature Publishing Group | Immunology | Scoop.it](https://img.scoop.it/UI_tJA6EvDLpgQ4qSgtkaDl72eJkfbmt4t8yenImKBVvK0kTmF0xjctABnaLJIm9)




Current Opinion in Immunology
Volume 33, April 2015, Pages 1–8
Lymphocyte development and activation * Tumour immunology
Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumorsCariad Chester1, Aurelien Marabelle2, Roch Houot3, 4, 5, Holbrook E Kohrt1, doi:10.1016/j.coi.2014.12.010
Volume 33, April 2015, Pages 1–8
Lymphocyte development and activation * Tumour immunology
Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumorsCariad Chester1, Aurelien Marabelle2, Roch Houot3, 4, 5, Holbrook E Kohrt1, doi:10.1016/j.coi.2014.12.010Get rights and content