Highlights
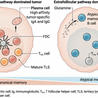
•CTLA-4-specific antibodies deplete intratumoral regulatory T cells via activating FcγRs.•Broadly neutralizing antibodies require activating FcγRs for their activity.•Agonistic antibodies require higher order crosslinking through the inhibitory FcγRIIb.•Organ-specific FcγRIIb positive cells provide higher order crosslinking for agonistic antibodies.
Given the widespread use of antibodies of the immunoglobulin G (IgG) class as cytotoxic, immunomodulatory, and neutralizing agents in the therapy of malignant, infectious, and autoimmune diseases, understanding the molecular and cellular mechanisms responsible for their therapeutic activity is of major importance. While Fcγ receptors (FcγR) have well-appreciated roles as effectors of cytotoxic IgG activity, it has only recently become clear that the functionality of immunomodulatory and neutralizing IgG preparations also depends on cellular FcγRs. Here, we review current models of IgG activity in infectious and inflammatory settings, and examine the importance of cell type-specific expression of FcγRs in determining functional outcome. We discuss how this knowledge may be used to improve the activity of therapeutic antibody preparations and outline important areas of focus for future research.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...






Trends Immunology
Volume 36, Issue 6, p325–336, June 2015Feature ReviewFcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activitiesFalk Nimmerjahn, Sina Gordan, Anja LuxInstitute of Genetics at the Department of Biology, Friedrich-Alexander University Erlangen-Nürnberg, Erwin-Rommelstrasse 3, 91058 Erlangen, Germany DOI: http://dx.doi.org/10.1016/j.it.2015.04.005 |