RT @genentech: New paper from our scientists in @Cancer_Cell shows how TIGIT may be potential #immunotherapy target http://t.co/FswC0GF9YB
Highlights
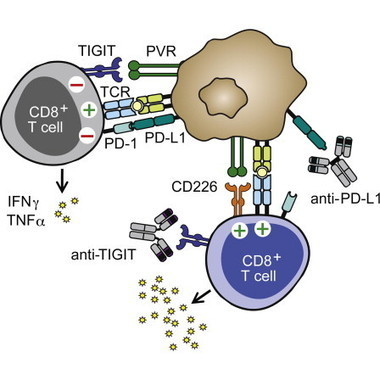
•Human and murine tumor-infiltrating CD8+ T cells express high levels of TIGIT•Antibody coblockade of TIGIT and PDL1 elicits tumor rejection in preclinical models•TIGIT selectively limits the effector function of chronically stimulated CD8+T cells•TIGIT interacts with CD226 in cis and disrupts CD226 homodimerization
Summary
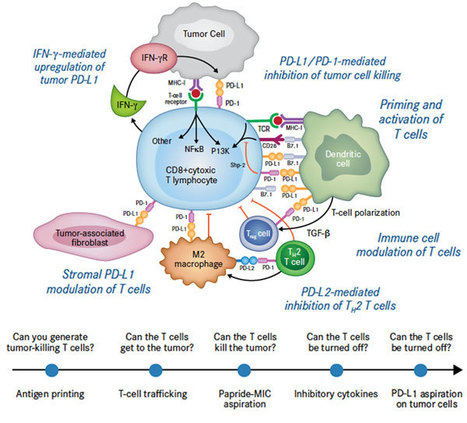
Tumors constitute highly suppressive microenvironments in which infiltrating T cells are “exhausted” by inhibitory receptors such as PD-1. Here we identify TIGIT as a coinhibitory receptor that critically limits antitumor and other CD8+ T cell-dependent chronic immune responses. TIGIT is highly expressed on human and murine tumor-infiltrating T cells, and, in models of both cancer and chronic viral infection, antibody coblockade of TIGIT and PD-L1 synergistically and specifically enhanced CD8+ T cell effector function, resulting in significant tumor and viral clearance, respectively. This effect was abrogated by blockade of TIGIT’s complementary costimulatory receptor, CD226, whose dimerization is disrupted upon direct interaction with TIGIT in cis. These results define a key role for TIGIT in inhibiting chronic CD8+ T cell-dependent responses.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...






Cancer Cell
The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8+ T Cell Effector FunctionRobert J. Johnston, Laetitia Comps-Agrar, Jason Hackney, Xin Yu, Mahrukh Huseni, Yagai Yang, Summer Park, Vincent Javinal, Henry Chiu, Bryan Irving,Dan L. Eaton, Jane L. Grogan DOI: http://dx.doi.org/10.1016/j.ccell.2014.10.018