•NFAT proteins induce a transcriptional program of CD8+ T cell exhaustion•CD8+ T cells lacking NFAT fail to express inhibitory surface receptors•An engineered NFAT that cannot cooperate with AP-1 strongly induces exhaustion•The engineered NFAT1 blunts TCR signaling and impairs CD8+ function in vivo
Summary
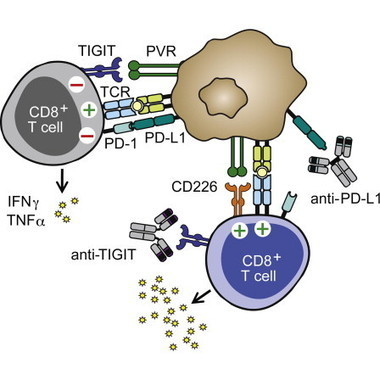
During persistent antigen stimulation, CD8+ T cells show a gradual decrease in effector function, referred to as exhaustion, which impairs responses in the setting of tumors and infections. Here we demonstrate that the transcription factor NFAT controls the program of T cell exhaustion. When expressed in cells, an engineered form of NFAT1 unable to interact with AP-1 transcription factors diminished T cell receptor (TCR) signaling, increased the expression of inhibitory cell surface receptors, and interfered with the ability of CD8+ T cells to protect against Listeriainfection and attenuate tumor growth in vivo. We defined the genomic regions occupied by endogenous and engineered NFAT1 in primary CD8+ T cells and showed that genes directly induced by the engineered NFAT1 overlapped with genes expressed in exhausted CD8+ T cells in vivo. Our data show that NFAT promotes T cell anergy and exhaustion by binding at sites that do not require cooperation with AP-1.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...









exhaustion explained?