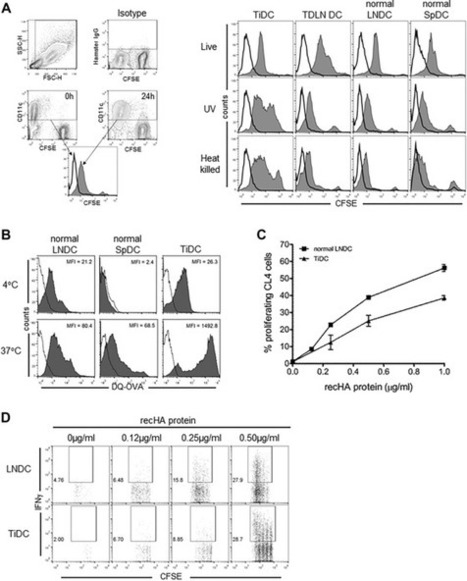
Highlights•Tumor-infiltrating myeloid cells link angiogenesis and immune tolerance to drive tumor growth.•Hypoxia fuels proangiogenic and immune-suppressive programming of myeloid cells in tumors.•Immune-suppressive myeloid cells can drive tumor resistance to antiangiogenic therapy.•Antiangiogenic therapy efficacy may partly depend on promoting an immune-stimulating environment.
Angiogenesis is a hallmark of cancer because its induction is indispensable to fuel an expanding tumor. The tumor microenvironment contributes to tumor vessel growth, and distinct myeloid cells recruited by the tumor have been shown not only to support angiogenesis but also to foster an immune suppressive environment that supports tumor expansion and progression. Recent findings suggest that the intertwined regulation of angiogenesis and immune modulation can offer therapeutic opportunities for the treatment of cancer. We review the mechanisms by which distinct myeloid cell populations contribute to tumor angiogenesis, discuss current approaches in the clinic that are targeting both angiogenic and immune suppressive pathways, and highlight important areas of future research.
Via Krishan Maggon


 Your new post is loading...
Your new post is loading...