Your new post is loading...
 Your new post is loading...
An ancient conflict between hosts and pathogens has driven the innate and adaptive arms of immunity. Knowledge about this interplay can not only help us identify biological mechanisms but also reveal pathogen vulnerabilities that can be leveraged therapeutically. The humoral response to SARS-CoV-2 infection has been the focus of intense research, and the role of the innate immune system has received significantly less attention. Here, we review current knowledge of the innate immune response to SARS-CoV-2 infection and the various means SARS-CoV-2 employs to evade innate defense systems. We also consider the role of innate immunity in SARS-CoV-2 vaccines and in the phenomenon of long COVID. Published in Cell. Mol. Immunology (Nov. 20, 2023): https://doi.org/10.1038/s41423-023-01104-y
Via Juan Lama
The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into human cells is an essential step for virus transmission and development of COVID 19. Although the lung epithelial cells are its initial target, SARS-CoV-2 also can infect endothelial cells. Endothelial cells are the major constituents of the vascular system and cardiovascular complication is a hallmark of severe COVID-19. Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV-2. However, the possible involvement of other cellular components in the viral entry is not fully understood. A team of researchers from the Boston University School of Medicine (BUSM) has identified extracellular vimentin as an attachment factor that facilitates SARS-CoV-2 entry into human cells. Vimentin is a structural protein that is widely expressed in the cells of mesenchymal origin such as endothelial cells and a potential novel target against SARS-CoV-2, which could block the infection of the SARS-CoV-2. "Severe endothelial injury, vascular thrombosis, and obstruction of alveolar capillaries (tiny air sacs scattered throughout the lungs) are common features of severe COVID-19. Identification of vimentin as a host attachment factor for SARS-CoV-2 can provide new insight into the mechanism of SARS-CoV-2 infection of the vascular system and can lead to the development of novel treatment strategies," said corresponding author Nader Rahimi, Ph.D., associate professor of pathology & laboratory medicine at BUSM. The researchers used liquid chromatography–tandem mass spectrometry (LC-MS/MS) and identified vimentin as a protein that binds to the SARS-CoV-2 spike (S) protein and facilitates SARS-CoV-2 infection. They also found that depletion of vimentin significantly reduces SARS-CoV-2 infection of human endothelial cells. In contrast, over-expression of vimentin with ACE2 significantly increased the infection rate. "More importantly, we saw that the CR3022 antibody inhibited the binding of vimentin with CoV-2-S-protein, and neutralized SARS-CoV-2 entry into human cells," explained Rahimi. Findings Published in P.N.A.S. (Jan. 25, 2022): https://doi.org/10.1073/pnas.2113874119
Via Juan Lama
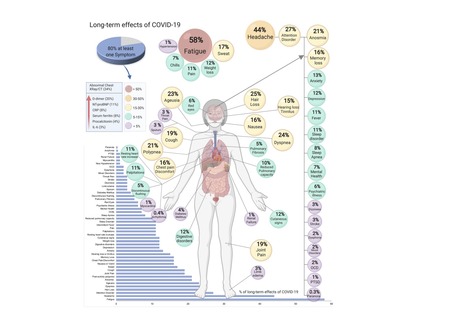
COVID-19, caused by SARS-CoV-2, can involve sequelae and other medical complications that last weeks to months after initial recovery, which has come to be called Long-COVID or COVID long-haulers. This systematic review and meta-analysis aims to identify studies assessing long-term effects of COVID-19 and estimates the prevalence of each symptom, sign, or laboratory parameter of patients at a post-COVID-19 stage. LitCOVID (PubMed and Medline) and Embase were searched by two independent researchers. All articles with original data for detecting long-term COVID-19 published before 1st of January 2021 and with a minimum of 100 patients were included. For effects reported in two or more studies, meta-analyses using a random-effects model were performed using the MetaXL software to estimate the pooled prevalence with 95% CI. Heterogeneity was assessed using I2 statistics. The Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) reporting guideline was followed. A total of 18,251 publications were identified, of which 15 met the inclusion criteria. The prevalence of 55 long-term effects was estimated, 21 meta-analyses were performed, and 47,910 patients were included. The follow-up time ranged from 15 to 110 days post-viral infection. The age of the study participants ranged between 17 and 87 years. It was estimated that 80% (95% CI 65-92) of the patients that were infected with SARS-CoV-2 developed one or more long-term symptoms. The five most common symptoms were fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%). All meta-analyses showed medium (n=2) to high heterogeneity (n=13). In order to have a better understanding, future studies need to stratify by sex, age, previous comorbidities, severity of COVID-19 (ranging from asymptomatic to severe), and duration of each symptom. From the clinical perspective, multi-disciplinary teams are crucial to developing preventive measures, rehabilitation techniques, and clinical management strategies with whole-patient perspectives designed to address long COVID-19 care. Preprint available in medRxiv (Jan. 30, 2021): https://doi.org/10.1101/2021.01.27.21250617
Via Juan Lama
The coronavirus disease 2019 (COVID-19) is determined by SARS-CoV-2 replication and host immune response, but studies evaluating viral evasion of immune response are lacking. Here we employed unbiased screening to identify SARS-CoV-2 proteins that antagonize type-I interferon (IFN-I) response. Three proteins were found to antagonize IFN-I production via distinct mechanisms: nsp6 binds TBK1 to suppress IRF3 phosphorylation; nsp13 binds and blocks TBK1 phosphorylation; and ORF6 binds importin KPNA2 to inhibit IRF3 nuclear translocation. Two sets of viral proteins were identified to antagonize IFN-I signaling through blocking STAT1/STAT2 phosphorylation or nuclear translocation. Remarkably, SARS-CoV-2 nsp1 and nsp6 suppressed IFN-I signaling more efficiently than SARS-CoV and MERS-CoV. Thus, when treated with IFN-I, a SARS-CoV2 replicon replicated to a higher level than chimeric replicons containing nsp1 or nsp6 from SARS-CoV or MERS-CoV. Altogether, the study has provided insights on SARS-CoV-2 evasion of IFN-I response and its potential impact on viral transmission and pathogenesis. Published in Cell Reports (September 19, 2020):
Via Juan Lama
|
NIH-funded study suggests need to boost CD8+ T cell response after infection. The magnitude and quality of a key immune cell’s response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection, a study has found. In addition, the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly, people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated. These findings, which suggest that the virus damages an important immune-cell response, were published today in the journal Immunity. The study was co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and led by Mark M. Davis, Ph.D. Dr. Davis is the director of the Stanford Institute for Immunity, Transplantation and Infection and a professor of microbiology and immunology at Stanford University School of Medicine in Palo Alto, California. He is also a Howard Hughes Medical Institute Investigator. Dr. Davis and colleagues designed a very sensitive tool to analyze how immune cells called CD4+ T cells and CD8+ T cells respond to SARS-CoV-2 infection and vaccination. These cells coordinate the immune system’s response to the virus and kill other cells that have been infected, helping prevent COVID-19. The tool was designed to identify T cells that target any of dozens of specific regions on the virus’s spike protein as well as some other viral regions. The Pfizer-BioNTech vaccine uses parts of the SARS-CoV-2 spike protein to elicit an immune response without causing infection. The investigators studied CD4+ and CD8+ T-cell responses in blood samples from three groups of volunteers. One group had never been infected with SARS-CoV-2 and received two doses of the Pfizer-BioNTech COVID-19 vaccine. The second group had previously been infected with SARS-CoV-2 and received two doses of the vaccine. The third group had COVID-19 and was unvaccinated. The researchers found that vaccination of people who had never been infected with SARS-CoV-2 induced robust CD4+ and CD8+ T-cell responses to the virus’ spike protein. In addition, these T cells produced multiple types of cell-signaling molecules called cytokines, which recruit other immune cells—including antibody-producing B cells—to fight pathogens. However, people who had been infected with SARS-CoV-2 prior to vaccination produced spike-specific CD8+ T cells at considerably lower levels—and with less functionality—than vaccinated people who had never been infected. Moreover, the researchers observed substantially lower levels of spike-specific CD8+ T cells in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Taken together, the investigators write, these findings suggest that SARS-CoV-2 infection damages the CD8+ T cell response, an effect akin to that observed in earlier studies showing long-term damage to the immune system after infection with viruses such as hepatitis C or HIV. The new findings highlight the need to develop vaccination strategies to specifically boost antiviral CD8+ T cell responses in people previously infected with SARS-CoV-2, the researchers conclude. Research published (March 15, 2023) in Immunity: https://doi.org/10.1016/j.immuni.2023.03.005
Via Juan Lama
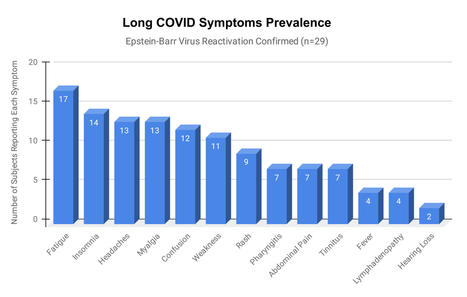
Epstein-Barr virus (EBV) reactivation resulting from the inflammatory response to coronavirus infection may be the cause of previously unexplained long COVID symptoms—such as fatigue, brain fog, and rashes—that occur in approximately 30% of patients after recovery from initial COVID-19 infection. The first evidence linking EBV reactivation to long COVID, as well as an analysis of long COVID prevalence, is outlined in a new long COVID study published in the journal Pathogens. "We ran EBV antibody tests on recovered COVID-19 patients, comparing EBV reactivation rates of those with long COVID symptoms to those without long COVID symptoms," said lead study author Jeffrey E. Gold of World Organization. "The majority of those with long COVID symptoms were positive for EBV reactivation, yet only 10% of controls indicated reactivation." The researchers began by surveying 185 randomly selected patients recovered from COVID-19 and found that 30.3% had long term symptoms consistent with long COVID after initial recovery from SARS-CoV-2 infection. This included several patients with initially asymptomatic COVID-19 cases who later went on to develop long COVID symptoms. The researchers then found, in a subset of 68 COVID-19 patients randomly selected from those surveyed, that 66.7% of long COVID subjects versus 10% of controls were positive for EBV reactivation based on positive EBV early antigen-diffuse (EA-D) IgG or EBV viral capsid antigen (VCA) IgM titers. The difference was significant (p < 0.001, Fisher's exact test). "We found similar rates of EBV reactivation in those who had long COVID symptoms for months, as in those with long COVID symptoms that began just weeks after testing positive for COVID-19," said coauthor David J. Hurley, Ph.D., a professor and molecular microbiologist at the University of Georgia. "This indicated to us that EBV reactivation likely occurs simultaneously or soon after COVID-19 infection." The relationship between SARS-CoV-2 and EBV reactivation described in this study opens up new possibilities for long COVID diagnosis and treatment. The researchers indicated that it may be prudent to test patients newly positive for COVID-19 for evidence of EBV reactivation indicated by positive EBV EA-D IgG, EBV VCA IgM, or serum EBV DNA tests. If patients show signs of EBV reactivation, they can be treated early to reduce the intensity and duration of EBV replication, which may help inhibit the development of long COVID. "As evidence mounts supporting a role for EBV reactivation in the clinical manifestation of acute COVID-19, this study further implicates EBV in the development of long COVID," said Lawrence S. Young, Ph.D., a virologist at the University of Warwick, and Editor-in-Chief of Pathogens. "If a direct role for EBV reactivation in long COVID is supported by further studies, this would provide opportunities to improve the rational diagnosis of this condition and to consider the therapeutic value of anti-herpesvirus agents such as ganciclovir." Original findings published in Pathogens (June 10, 2021): https://doi.org/10.3390/pathogens10060763
Via Juan Lama
A provocative study suggests that certain colds may leave antibodies against the new coronavirus, perhaps explaining why children are more protected than adults. It’s been a big puzzle of the pandemic: Why are children so much less likely than adults to become infected with the new coronavirus and, if infected, less likely to become ill? A possible reason may be that many children already have antibodies to other coronaviruses, according to researchers at the Francis Crick Institute in London. About one in five of the colds that plague children are caused by viruses in this family. Antibodies to those viruses may also block SARS-CoV-2, the new coronavirus causing the pandemic. In a study published Friday in Science, the group, led by George Kassiotis, who heads the Retroviral Immunology Laboratory at the institute, reports that on average only 5 percent of adults had these antibodies, but 43 percent of children did. Researchers who did not participate in the study were intrigued by the finding. H. Benjamin Larman, an immunologist at Johns Hopkins School of Medicine, called it a “well-done study that puts forward a compelling theory which is supported by their data.” Stephen J. Elledge, a genetics professor at Harvard Medical School and Brigham and Women’s Hospital, had a similar response. He and others have found many people have antibodies to common colds caused by other coronaviruses; in laboratory studies, these antibodies also block the new coronavirus. In March, as the pandemic was just beginning, Dr. Kassiotis and his colleagues decided to develop a highly sensitive antibody test. To assess it, they examined blood samples taken before the pandemic from over 300 adults and 48 children and adolescents, comparing them with samples from more than 170 people who had been infected with the new coronavirus. The scientists expected samples taken before the pandemic to have no antibodies that attacked the new coronavirus. Those were to be the controls for the test the scientists were developing. Instead, they found that many children, and some adults, carried one antibody in particular that can prevent coronaviruses, including the new one, from entering cells. This antibody attaches itself to a spike that pokes out of coronaviruses. While the tip of the spike is unique to the new coronavirus, the base is found in all coronaviruses, Dr. Kassiotis said. In lab tests, antibodies to the base of the spike prevented the new coronavirus from entering cells in order to reproduce. Now the researchers are planning to expand their study to monitor thousands of children and adults. Some have antibodies that can block the new coronavirus in lab tests. Others do not. “If they have the pandemic strain, are they protected?” Dr. Kassiotis asked. Will they get sick, he wondered, or will the infection be all but undetectable? Dr. Elledge and his colleagues at Harvard developed their own highly specific, sensitive and exhaustive antibody test, VirScan. It is able to detect a diverse collection of antibodies with that are directed at any of more than 800 places on the new coronavirus, including the antibody that Dr. Kassiotis and his colleagues studied. After examining blood taken from 190 people before the pandemic emerged, Dr. Elledge and his colleagues concluded that many already had antibodies, including the one targeting the base of the spike — presumably from infections with related coronaviruses that cause colds... Cited study published in Science (Nov. 6, 2020): https://doi.org/10.1126/science.abe1107
Via Juan Lama
|


 Your new post is loading...
Your new post is loading...