Your new post is loading...
 Your new post is loading...
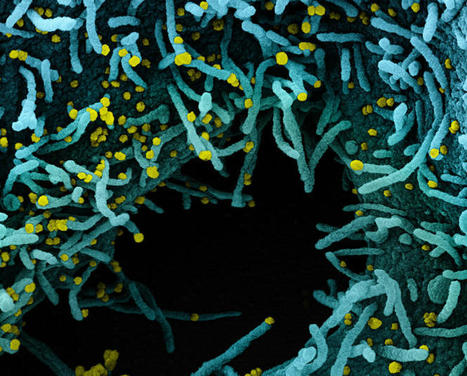
An ancient conflict between hosts and pathogens has driven the innate and adaptive arms of immunity. Knowledge about this interplay can not only help us identify biological mechanisms but also reveal pathogen vulnerabilities that can be leveraged therapeutically. The humoral response to SARS-CoV-2 infection has been the focus of intense research, and the role of the innate immune system has received significantly less attention. Here, we review current knowledge of the innate immune response to SARS-CoV-2 infection and the various means SARS-CoV-2 employs to evade innate defense systems. We also consider the role of innate immunity in SARS-CoV-2 vaccines and in the phenomenon of long COVID. Published in Cell. Mol. Immunology (Nov. 20, 2023): https://doi.org/10.1038/s41423-023-01104-y
Via Juan Lama
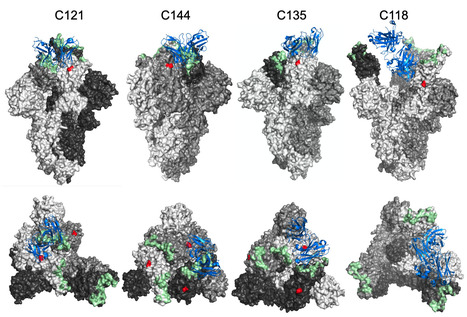
The glycosylation of viral envelope proteins can play important roles in virus biology and immune evasion. The spike (S) glycoprotein of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) includes 22 N-linked glycosylation sequons and 17 O-linked glycosites. Here, we investigated the effect of individual glycosylation sites on SARS-CoV-2 S function in pseudotyped virus infection assays and on sensitivity to monoclonal and polyclonal neutralizing antibodies. In most cases, removal of individual glycosylation sites decreased the infectiousness of the pseudotyped virus. For glycosylation mutants in the N-terminal domain (NTD) and the receptor binding domain (RBD), reduction in pseudotype infectivity was predicted by a commensurate reduction in the level of virion-incorporated spike protein. Notably, the presence of a glycan at position N343 within the RBD had diverse effects on neutralization by RBD-specific monoclonal antibodies (mAbs) cloned from convalescent individuals. The N343 glycan reduced overall sensitivity to polyclonal antibodies in plasma from COVID-19 convalescent individuals, suggesting a role for SARS-CoV-2 spike glycosylation in immune evasion. However, vaccination of convalescent individuals produced neutralizing activity that was resilient to the inhibitory effect of the N343 glycan. Preprint at bioRxiv (June 30, 2023): https://doi.org/10.1101/2023.06.30.547241
Via Juan Lama
interferon, friend or foe
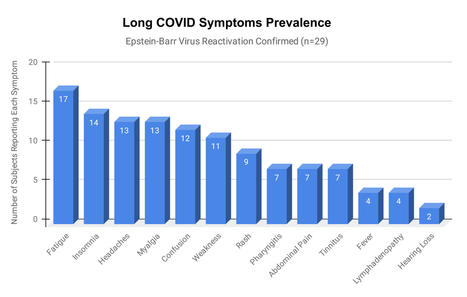
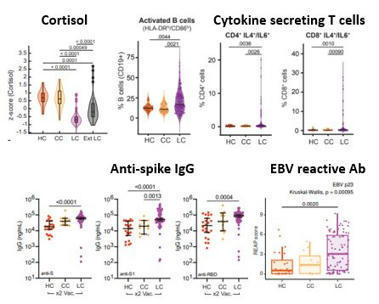
Epstein-Barr virus (EBV) reactivation resulting from the inflammatory response to coronavirus infection may be the cause of previously unexplained long COVID symptoms—such as fatigue, brain fog, and rashes—that occur in approximately 30% of patients after recovery from initial COVID-19 infection. The first evidence linking EBV reactivation to long COVID, as well as an analysis of long COVID prevalence, is outlined in a new long COVID study published in the journal Pathogens. "We ran EBV antibody tests on recovered COVID-19 patients, comparing EBV reactivation rates of those with long COVID symptoms to those without long COVID symptoms," said lead study author Jeffrey E. Gold of World Organization. "The majority of those with long COVID symptoms were positive for EBV reactivation, yet only 10% of controls indicated reactivation." The researchers began by surveying 185 randomly selected patients recovered from COVID-19 and found that 30.3% had long term symptoms consistent with long COVID after initial recovery from SARS-CoV-2 infection. This included several patients with initially asymptomatic COVID-19 cases who later went on to develop long COVID symptoms. The researchers then found, in a subset of 68 COVID-19 patients randomly selected from those surveyed, that 66.7% of long COVID subjects versus 10% of controls were positive for EBV reactivation based on positive EBV early antigen-diffuse (EA-D) IgG or EBV viral capsid antigen (VCA) IgM titers. The difference was significant (p < 0.001, Fisher's exact test). "We found similar rates of EBV reactivation in those who had long COVID symptoms for months, as in those with long COVID symptoms that began just weeks after testing positive for COVID-19," said coauthor David J. Hurley, Ph.D., a professor and molecular microbiologist at the University of Georgia. "This indicated to us that EBV reactivation likely occurs simultaneously or soon after COVID-19 infection." The relationship between SARS-CoV-2 and EBV reactivation described in this study opens up new possibilities for long COVID diagnosis and treatment. The researchers indicated that it may be prudent to test patients newly positive for COVID-19 for evidence of EBV reactivation indicated by positive EBV EA-D IgG, EBV VCA IgM, or serum EBV DNA tests. If patients show signs of EBV reactivation, they can be treated early to reduce the intensity and duration of EBV replication, which may help inhibit the development of long COVID. "As evidence mounts supporting a role for EBV reactivation in the clinical manifestation of acute COVID-19, this study further implicates EBV in the development of long COVID," said Lawrence S. Young, Ph.D., a virologist at the University of Warwick, and Editor-in-Chief of Pathogens. "If a direct role for EBV reactivation in long COVID is supported by further studies, this would provide opportunities to improve the rational diagnosis of this condition and to consider the therapeutic value of anti-herpesvirus agents such as ganciclovir." Original findings published in Pathogens (June 10, 2021): https://doi.org/10.3390/pathogens10060763
Via Juan Lama
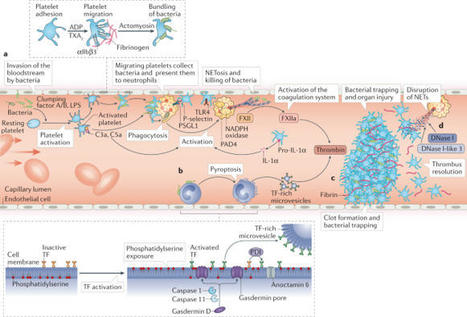
Thrombosis is the most feared complication of cardiovascular diseases and a main cause of death worldwide, making it a major health-care challenge. Platelets and the coagulation cascade are effectively targeted by antithrombotic approaches, which carry an inherent risk of bleeding. Moreover, antithrombotics cannot completely prevent thrombotic events, implicating a therapeutic gap due to a third, not yet adequately addressed mechanism, namely inflammation. In this Review, we discuss how the synergy between inflammation and thrombosis drives thrombotic diseases. We focus on the huge potential of anti-inflammatory strategies to target cardiovascular pathologies. Findings in the past decade have uncovered a sophisticated connection between innate immunity, platelet activation and coagulation, termed immunothrombosis. Immunothrombosis is an important host defence mechanism to limit systemic spreading of pathogens through the bloodstream. However, the aberrant activation of immunothrombosis in cardiovascular diseases causes myocardial infarction, stroke and venous thromboembolism. The clinical relevance of aberrant immunothrombosis, referred to as thromboinflammation, is supported by the increased risk of cardiovascular events in patients with inflammatory diseases but also during infections, including in COVID-19. Clinical trials in the past 4 years have confirmed the anti-ischaemic effects of anti-inflammatory strategies, backing the concept of a prothrombotic function of inflammation. Targeting inflammation to prevent thrombosis leaves haemostasis mainly unaffected, circumventing the risk of bleeding associated with current approaches. Considering the growing number of anti-inflammatory therapies, it is crucial to appreciate their potential in covering therapeutic gaps in cardiovascular diseases. In this Review, Stark and Massberg discuss how the interplay between innate immunity, platelet activation and coagulation, known as immunothrombosis, functions as a host defence mechanism to limit pathogen spreading, yet its aberrant activation, termed thromboinflammation, results in thrombotic complications, highlighting the therapeutic potential of anti-inflammatory strategies in cardiovascular pathologies.
A previous history of SARS-CoV-2 infection was associated with an 84% lower risk of
infection, with median protective effect observed 7 months following primary infection.
This time period is the minimum probable effect because seroconversions were not included.
This study shows that previous infection with SARS-CoV-2 induces effective immunity
to future infections in most individuals.
High levels of soluble CD25 in COVID‐19 patients were found being associated with severe disease and prolonged viral shedding. We discovered that the elevation of sCD25 was caused by th
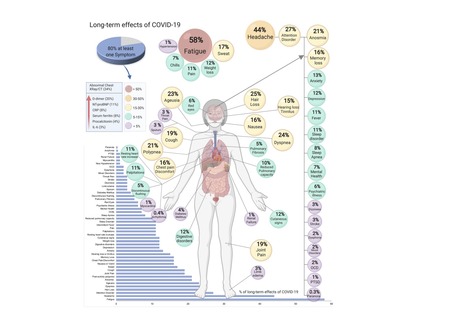
COVID-19, caused by SARS-CoV-2, can involve sequelae and other medical complications that last weeks to months after initial recovery, which has come to be called Long-COVID or COVID long-haulers. This systematic review and meta-analysis aims to identify studies assessing long-term effects of COVID-19 and estimates the prevalence of each symptom, sign, or laboratory parameter of patients at a post-COVID-19 stage. LitCOVID (PubMed and Medline) and Embase were searched by two independent researchers. All articles with original data for detecting long-term COVID-19 published before 1st of January 2021 and with a minimum of 100 patients were included. For effects reported in two or more studies, meta-analyses using a random-effects model were performed using the MetaXL software to estimate the pooled prevalence with 95% CI. Heterogeneity was assessed using I2 statistics. The Preferred Reporting Items for Systematic Reviewers and Meta-analysis (PRISMA) reporting guideline was followed. A total of 18,251 publications were identified, of which 15 met the inclusion criteria. The prevalence of 55 long-term effects was estimated, 21 meta-analyses were performed, and 47,910 patients were included. The follow-up time ranged from 15 to 110 days post-viral infection. The age of the study participants ranged between 17 and 87 years. It was estimated that 80% (95% CI 65-92) of the patients that were infected with SARS-CoV-2 developed one or more long-term symptoms. The five most common symptoms were fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%). All meta-analyses showed medium (n=2) to high heterogeneity (n=13). In order to have a better understanding, future studies need to stratify by sex, age, previous comorbidities, severity of COVID-19 (ranging from asymptomatic to severe), and duration of each symptom. From the clinical perspective, multi-disciplinary teams are crucial to developing preventive measures, rehabilitation techniques, and clinical management strategies with whole-patient perspectives designed to address long COVID-19 care. Preprint available in medRxiv (Jan. 30, 2021): https://doi.org/10.1101/2021.01.27.21250617
Via Juan Lama
Cytokine Storm Cytokine storm, a life-threatening disorder involving cytokine elevations and immune-cell hyperactivation, has various causes and is characterized by constitutional symptoms
Via Krishan Maggon
Shomuradova et al. assessed the immune response to SARS-CoV-2 in convalescent patients
and healthy donors. Antigen-specific T cells were increased in convalescents and in
donors sampled during the pandemic. The work identified two public epitopes from S-glycoprotein.
T cell receptor repertoire profiling of S-glycoprotein specific lymphocytes revealed
public CDR3 motifs.
Click on the article title to read more.
A provocative study suggests that certain colds may leave antibodies against the new coronavirus, perhaps explaining why children are more protected than adults. It’s been a big puzzle of the pandemic: Why are children so much less likely than adults to become infected with the new coronavirus and, if infected, less likely to become ill? A possible reason may be that many children already have antibodies to other coronaviruses, according to researchers at the Francis Crick Institute in London. About one in five of the colds that plague children are caused by viruses in this family. Antibodies to those viruses may also block SARS-CoV-2, the new coronavirus causing the pandemic. In a study published Friday in Science, the group, led by George Kassiotis, who heads the Retroviral Immunology Laboratory at the institute, reports that on average only 5 percent of adults had these antibodies, but 43 percent of children did. Researchers who did not participate in the study were intrigued by the finding. H. Benjamin Larman, an immunologist at Johns Hopkins School of Medicine, called it a “well-done study that puts forward a compelling theory which is supported by their data.” Stephen J. Elledge, a genetics professor at Harvard Medical School and Brigham and Women’s Hospital, had a similar response. He and others have found many people have antibodies to common colds caused by other coronaviruses; in laboratory studies, these antibodies also block the new coronavirus. In March, as the pandemic was just beginning, Dr. Kassiotis and his colleagues decided to develop a highly sensitive antibody test. To assess it, they examined blood samples taken before the pandemic from over 300 adults and 48 children and adolescents, comparing them with samples from more than 170 people who had been infected with the new coronavirus. The scientists expected samples taken before the pandemic to have no antibodies that attacked the new coronavirus. Those were to be the controls for the test the scientists were developing. Instead, they found that many children, and some adults, carried one antibody in particular that can prevent coronaviruses, including the new one, from entering cells. This antibody attaches itself to a spike that pokes out of coronaviruses. While the tip of the spike is unique to the new coronavirus, the base is found in all coronaviruses, Dr. Kassiotis said. In lab tests, antibodies to the base of the spike prevented the new coronavirus from entering cells in order to reproduce. Now the researchers are planning to expand their study to monitor thousands of children and adults. Some have antibodies that can block the new coronavirus in lab tests. Others do not. “If they have the pandemic strain, are they protected?” Dr. Kassiotis asked. Will they get sick, he wondered, or will the infection be all but undetectable? Dr. Elledge and his colleagues at Harvard developed their own highly specific, sensitive and exhaustive antibody test, VirScan. It is able to detect a diverse collection of antibodies with that are directed at any of more than 800 places on the new coronavirus, including the antibody that Dr. Kassiotis and his colleagues studied. After examining blood taken from 190 people before the pandemic emerged, Dr. Elledge and his colleagues concluded that many already had antibodies, including the one targeting the base of the spike — presumably from infections with related coronaviruses that cause colds... Cited study published in Science (Nov. 6, 2020): https://doi.org/10.1126/science.abe1107
Via Juan Lama
|
Celles et ceux qui me font l'honneur de me suivre se souviennent peut-être d'un récent post annonçant la création par Yale University du Centre de l'Infection…
NIH-funded study suggests need to boost CD8+ T cell response after infection. The magnitude and quality of a key immune cell’s response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection, a study has found. In addition, the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly, people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated. These findings, which suggest that the virus damages an important immune-cell response, were published today in the journal Immunity. The study was co-funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and led by Mark M. Davis, Ph.D. Dr. Davis is the director of the Stanford Institute for Immunity, Transplantation and Infection and a professor of microbiology and immunology at Stanford University School of Medicine in Palo Alto, California. He is also a Howard Hughes Medical Institute Investigator. Dr. Davis and colleagues designed a very sensitive tool to analyze how immune cells called CD4+ T cells and CD8+ T cells respond to SARS-CoV-2 infection and vaccination. These cells coordinate the immune system’s response to the virus and kill other cells that have been infected, helping prevent COVID-19. The tool was designed to identify T cells that target any of dozens of specific regions on the virus’s spike protein as well as some other viral regions. The Pfizer-BioNTech vaccine uses parts of the SARS-CoV-2 spike protein to elicit an immune response without causing infection. The investigators studied CD4+ and CD8+ T-cell responses in blood samples from three groups of volunteers. One group had never been infected with SARS-CoV-2 and received two doses of the Pfizer-BioNTech COVID-19 vaccine. The second group had previously been infected with SARS-CoV-2 and received two doses of the vaccine. The third group had COVID-19 and was unvaccinated. The researchers found that vaccination of people who had never been infected with SARS-CoV-2 induced robust CD4+ and CD8+ T-cell responses to the virus’ spike protein. In addition, these T cells produced multiple types of cell-signaling molecules called cytokines, which recruit other immune cells—including antibody-producing B cells—to fight pathogens. However, people who had been infected with SARS-CoV-2 prior to vaccination produced spike-specific CD8+ T cells at considerably lower levels—and with less functionality—than vaccinated people who had never been infected. Moreover, the researchers observed substantially lower levels of spike-specific CD8+ T cells in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Taken together, the investigators write, these findings suggest that SARS-CoV-2 infection damages the CD8+ T cell response, an effect akin to that observed in earlier studies showing long-term damage to the immune system after infection with viruses such as hepatitis C or HIV. The new findings highlight the need to develop vaccination strategies to specifically boost antiviral CD8+ T cell responses in people previously infected with SARS-CoV-2, the researchers conclude. Research published (March 15, 2023) in Immunity: https://doi.org/10.1016/j.immuni.2023.03.005
Via Juan Lama
5 juillet 2021 Une équipe de scientifiques internationaux a récemment identifié des anticorps ultrapuissants anti-coronavirus 2 (SRAS-CoV-2) anti-syndrome respiratoire aigu sévère provenant de donneurs convalescents. Les anticorps sont capables de neutraliser une large gamme de variantes du SRAS-CoV-2, même à des concentrations sous-nanomolaires. De plus, les combinaisons de ces anticorps réduisent le risque de générer des mutants d'échappement in vitro. L'étude a été publiée le 13 août 2021 dans la revue Science. https://www.science.org/doi/full/10.112 ... ce.abh1766 Sur le fond Le coronavirus 2 du syndrome respiratoire aigu sévère (SRAS-CoV-2), l'agent pathogène responsable de la maladie à coronavirus 2019 (COVID-19), est un virus à ARN simple brin enveloppé de sens positif appartenant à la famille des bêta-coronavirus humains. La glycoprotéine de pointe sur l'enveloppe virale est composée de deux sous-unités S1 et S2. Dont, la sous-unité S1 se lie directement au récepteur de l'enzyme de conversion de l'angiotensine 2 (ACE2) de la cellule hôte via le domaine de liaison au récepteur (RBD) pour initier le processus d'entrée virale. La majorité des anticorps thérapeutiques contre le SRAS-CoV-2 ont été conçus sur la base de la séquence de protéine de pointe native trouvée dans la souche Wuhan originale du SRAS-CoV-2. Ainsi, de nouvelles variantes virales avec de multiples mutations de la protéine de pointe peuvent probablement développer une résistance contre ces anticorps. Dans ce contexte, des études ont montré que les anticorps développés en réponse aux vaccins COVID-19 actuellement disponibles ont moins d'efficacité pour neutraliser les nouvelles variantes préoccupantes (COV) du SRAS-CoV, notamment B.1.1.7, B.1.351, P1 et B.1.617.2. Dans la présente étude, les scientifiques ont isolé et caractérisé des anticorps anti-pic RBD de patients guéris du COVID-19. Identification des anticorps Les anticorps ont été isolés de quatre donneurs convalescents infectés par la souche Washington-1 (WA-1) du SRAS-CoV-2. La séquence de pointe dans la souche WA-1 est similaire à la séquence de pointe dans la souche originale de Wuhan. Les cellules B isolées à partir d'échantillons de sang provenant de donneurs ont été triées pour l'identification des anticorps. Cela a conduit à l'identification de quatre anticorps neutralisants puissants ciblant le pic RBD. Ces anticorps ont montré une forte affinité pour le pic SRAS-CoV-2 même à des concentrations nanomolaires. Pour déterminer si les anticorps hautement puissants pouvaient bloquer l'ACE2 - la liaison aux pointes, des tests d'interférométrie par compétition ACE2 et de liaison à la surface cellulaire ont été effectués. Les résultats ont révélé que sur 4 anticorps, deux liés aux RBD en « position haute » et deux liés aux RBD en « position basse ». De plus, trois anticorps sur quatre bloquaient directement l'interaction RBD - ACE2, et un inhibait indirectement l'interaction par encombrement stérique - le ralentissement des réactions chimiques dû à l'encombrement stérique. Neutralisation médiée par les anticorps Tous les anticorps expérimentaux ont montré une puissance significativement plus élevée dans la neutralisation des variants contenant la mutation D614G que la souche WA-1. Une analyse plus poussée avec des particules lentivirales pseudotypées avec des variantes à pointes a indiqué que les anticorps maintiennent une puissance élevée pour neutraliser un ensemble diversifié de 10 variantes à pointes. Il est important de noter que trois des quatre anticorps expérimentaux ont montré une grande efficacité pour neutraliser 13 variantes circulantes préoccupantes/intéressantes du SRAS-CoV-2, notamment B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, P.1, P.2, B.1.617.1 et B.1.617.2. Analyse structurale et fonctionnelle des anticorps Des analyses au microscope cryoélectronique des structures du complexe anticorps-antigène ont révélé que deux anticorps avec le pouvoir de neutralisation le plus élevé se lient à la protéine de pointe avec tous les RBD en « position haute ». D'autres analyses structurelles ont révélé que les modes de liaison à l'épitope des anticorps sont responsables d'un pouvoir neutralisant élevé contre les COV du SRAS-CoV-2. La capacité de liaison et de neutralisation des anticorps a été affectée négativement par trois mutations de pointe, dont F486R, N487R et Y489R. Résistance aux anticorps Une pression de sélection d'anticorps a été appliquée à la souche WA-1 pour identifier les mutations potentielles d'échappement qui peuvent apparaître au cours de l'infection virale. La pression de sélection positive a été appliquée en incubant le virus avec des concentrations croissantes des anticorps pour déclencher la résistance aux anticorps. Dans deux des anticorps les plus puissants, l'un était affecté négativement par une seule mutation F486S et l'autre était affecté par les mutations F486L, N487D et Q493R. Cependant, la mutation Q493R a montré un impact négligeable sur la liaison et la neutralisation. Une analyse plus poussée a révélé que ces mutations d'échappement sont principalement absentes dans les variantes virales circulantes, indiquant l'absence de pression de sélection. En effectuant plusieurs cycles de sélection à l'aide de traitements combinés avec deux anticorps, il a été observé que les combinaisons d'anticorps pourraient réduire le risque d'acquisition de mutations d'échappement et le développement ultérieur de variantes virales résistantes. Source: News Medical et merci à QcLiLi, dans Twitter En référence à "Wang L. 2021. Anticorps ultrapuissants contre des variantes diverses et hautement transmissibles du SRAS-CoV-2. Sciences". -- -- --
Les variants qui apparaissent toujours plus différenciés et plus tenaces sont-ils un péril pour la sortie du tunnel ?
Emerging studies indicate that some coronavirus disease 2019 (COVID-19) patients suffer from persistent symptoms, including breathlessness and chronic…
Since the initial reports of a cluster of pneumonia cases of unidentified origin in Wuhan, China, in December 2019, the novel coronavirus that causes this disease — severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) — has spread throughout the world, igniting the twenty-first century’s deadliest pandemic. Over the past 12 months, a dizzying array of information has emerged from numerous laboratories, covering everything from the putative origin of SARS-CoV-2 to the development of numerous candidate vaccines. Many immunologists quickly pivoted from their existing research to focus on coronavirus disease 2019 (COVID-19) and, owing to this unprecedented convergence of efforts on one viral infection, a remarkable body of work has been produced and disseminated, through both preprint servers and peer-reviewed journals. Here, we take readers through the timeline of key discoveries during the first year of the pandemic, which showcases the extraordinary leaps in our understanding of the immune response to SARS-CoV-2 and highlights gaps in our knowledge as well as areas for future investigations. A timeline of the major scientific discoveries during the first year of the COVID-19 pandemic showcases the collaborative efforts that enabled the key aspects of the immune response to SARS-CoV-2 to be reported at unprecedented speed.
Scientists across the world are closely tracking the spread of mutations in the coronavirus and investigating whether they could render current vaccines less effective. SARS-CoV-2 is no Ferrari among viruses when it comes to mutations. Scientists reckon that its 30,000-base RNA genome acquires around two single-letter mutations a month, a rate around half as fast as influenza and one-quarter the rate of HIV. But allowed to multiply and jump from body to body for more than a year, SARS-CoV-2 has inevitably flourished into a genetically diverse tree branching into countless different variants. Many variants—defined by a specific assortment of mutations—are relatively unremarkable. But scientists have been keeping a close watch on three rapidly spreading variants—first identified in the UK, South Africa, and Brazil—which harbor an unusual constellation of mutations. They all share a mutation called N501Y that affects the receptor binding domain (RBD) of the spike protein, which the virus uses to clasp onto human cells’ receptors and enter them. That mutation replaces SARS-CoV-2’s 501st amino acid, asparagine, with tyrosine, potentially allowing it to bind more tightly to ACE2 receptors, studies in cells and animal models suggest. By itself, that mutation isn’t unusual, but the variants possess an exceptionally large number of other mutations, some also on the spike protein. Substantive changes to a virus’ behavior, such as heightened transmissibility, are likely the result of multiple mutations rather than individual ones, molecular epidemiologist Emma Hodcroft of the University of Bern tells The Atlantic. The observation that similar mutations have appeared in three independent variants, and the fact that they are spreading, makes scientists suspect that they may have an evolutionary edge. “They have multiple, eight to ten, mutations in the spike protein all stacked up at once—that suggests that there [is] a lot of evolution and adaption of the protein happening,” Daniel Jones, a molecular pathologist at the Ohio State University, tells The Scientist. “The concern being that since that’s the target of vaccinations and . . . the target for antibody [therapies] like the Regeneron cocktail, for instance, that it might be the beginning of a virus that could evade antibody therapy and/or vaccine coverage.” SARS-CoV-2 mutations’ effects on transmissibility It’s often in a virus’ interest to become more transmissible so it can spread and replicate more quickly. Earlier in the pandemic, a spike protein mutation known as D614G—which is widely believed to have made the virus more transmissible—surged to dominance around the world, notes virologist John Moore of Weill Cornell Medical College. Epidemiological data suggest that the B.1.1.7 variant, a descendant of the D614G lineage first identified in the UK that has spread to other parts of the world, also has heightened transmissibility. Eight of the 17 mutations it has recently accumulated are in the spike protein, which could feasibly have an effect on ACE2 binding and virus replication. Hypothetically, if a virus can bind more tightly to the body’s ACE2 receptors, it could be more capable of establishing an infection once it gets into the body and/or of generating more viral particles in the upper respiratory tract, making it easier to transmit to other people, particularly during the presymptomatic stage, explains Theodora Hatziioannou, a virologist at the Rockefeller University in New York. She adds that in her view, it’s hard to definitively ascribe case surges, including the current one in the UK, to single factors such as increased transmissibility, over other driving factors, such as what she sees as ineffective lockdown policies. “I’m not saying that [increased] transmissibility is out of the question. I’m just saying it’s extremely hard to prove.” In South Africa, epidemiologists have estimated that the new variant there, B.1.351 (also known as 501Y.V2), is around 50 percent more contagious compared with dominant lineages, based on its rapid spread, according to The Wall Street Journal. In Brazil, it’s too early to conclude whether a variant now circulating there, called P.1, is inherently more transmissible. First reported on January 12 in the state of Amazonas, it’s been associated with a devastating surge in cases in Manaus, a city where researchers had previously estimated that 75 percent of residents had already been infected with SARS-CoV-2. But it’s still unclear whether properties of the virus itself are contributing to the surge, says virologist Paola Resende of the Oswaldo Cruz Institute in Rio de Janeiro. “In Brazil, we can see a lot of parties, we can see the pubs crowded, and people are on the streets not wearing masks. I think this behavior of the population is the main reason [for] the increase.” Evading the immune system Our immune system—and, in particular, antibodies—is a powerful evolutionary force on viruses. Some pathogens such as influenza, and maybe also common cold-causing coronaviruses, mutate their proteins toward new shapes to avoid being targeted by antibodies that would normally prevent them from infecting cells, a process known as antigenic drift. A study recently posted as a preprint to bioRxiv by Hatziioannou and her colleagues suggests that the RBD mutations present in the B.1.351 variant are due to antigenic drift. The team passaged a model virus bearing the dominant SARS-CoV-2 spike protein in the presence of individual neutralizing antibodies extracted from people who had received either the Moderna or Pfizer/BioNTech vaccine. Depending on which antibody they were cultured with, the viruses would gradually adopt a single mutation—either E484K, K417N, and N501Y—which are present in B.1.351. That suggests that “the virus is mutating in these positions to avoid antibodies,” Hatziioannou says. Such antibody-escape mutations don’t necessarily mean that the virus will cause more severe disease or entirely outwit the immune response, she cautions. There are other parts of the immune system to help clear the virus. There’s no evidence so far to suggest that the variants identified in South Africa or Brazil are more lethal. Based on an analysis of several datasets, scientists in the UK suggested last week there’s a “realistic possibility” that B.1.1.7 is deadlier than previous strains, but experts say it’s still too early to draw that conclusion. Another concern is about whether people who have overcome mild infections with older variants could become reinfected with a new one. Nobody that I know has been losing a moment of sleep over the UK variant from a vaccine-efficacy perspective.....
Via Juan Lama
On 12 March 2020, the outbreak of coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization. As of 4 August 2020, more than 18 million confirmed infections had been reported globally. Most patients have mild symptoms, but some patients develop respiratory failure which is the leading cause of death among COVID-19 patients. Endothelial cells with high levels of angiotensin-converting enzyme 2 expression are major participants and regulators of inflammatory reactions and coagulation. Accumulating evidence suggests that endothelial activation and dysfunction participate in COVID-19 pathogenesis by altering the integrity of vessel barrier, promoting pro-coagulative state, inducing endothelial inflammation, and even mediating leukocyte infiltration. This review describes the proposed cellular and molecular mechanisms of endothelial activation and dysfunction during COVID-19 emphasizing the principal mediators and therapeutic implications.
Home/Newsroom/Q&A Detail/Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19 Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19 15 October 2020 | Q&A What is ‘herd immunity’? ‘Herd immunity’, also known as ‘population immunity’, is a concept used for vaccination, in which a population can be protected from a certain virus if a threshold of vaccination is reached. Herd immunity is achieved by protecting people from a virus, not by exposing them to it. Vaccines train our immune systems to create proteins that fight disease, known as ‘antibodies’, just as would happen when we are exposed to a disease but – crucially – vaccines work without making us sick. Vaccinated people are protected from getting the disease in question and passing it on, breaking any chains of transmission. Visit our webpage on COVID-19 and vaccines for more detail. With herd immunity, the vast majority of a population are vaccinated, lowering the overall amount of virus able to spread in the whole population. As a result, not every single person needs to be vaccinated to be protected, which helps ensure vulnerable groups who cannot get vaccinated are kept safe. The percentage of people who need to have antibodies in order to achieve herd immunity against a particular disease varies with each disease. For example, herd immunity against measles requires about 95% of a population to be vaccinated. The remaining 5% will be protected by the fact that measles will not spread among those who are vaccinated. For polio, the threshold is about 80%. Achieving herd immunity with safe and effective vaccines makes diseases rarer and saves lives. Find out more about the science behind herd immunity by watching or reading this interview with WHO’s Chief Scientist, Dr Soumya Swaminathan. Attempts to reach ‘herd immunity’ through exposing people to a virus are scientifically problematic and unethical. Letting COVID-19 spread through populations, of any age or health status will lead to unnecessary infections, suffering and death. The vast majority of people in most countries remain susceptible to this virus. Seroprevalence surveys suggest that in most countries, less than 10% of the population have been infected with COVID-19. We are still learning about immunity to COVID-19. Most people who are infected with COVID-19 develop an immune response within the first few weeks, but we don’t know how strong or lasting that immune response is, or how it differs for different people. There have also been reports of people infected with COVID-19 for a second time. Until we better understand COVID-19 immunity, it will not be possible to know how much of a population is immune and how long that immunity last for, let alone make future predictions. These challenges should preclude any plans that try to increase immunity within a population by allowing people to get infected. Although older people and those with underlying conditions are most at risk of severe disease and death, they are not the only ones at risk. Finally, while most infected people get mild or moderate forms of COVID-19 and some experience no disease, many become seriously ill and must be admitted into hospital. We are only beginning to understand the long-term health impacts among people who have had COVID-19, including what is being described as ‘Long COVID.’ WHO is working with clinicians and patient groups to better understand the long term effects of COVID-19. Read the Director-General’s opening remarks at the 12 October COVID-19 briefing for a summary of WHO’s position. Most people who are infected with COVID-19 develop an immune response within the first few weeks after infection. Research is still ongoing into how strong that protection is and how long it lasts. WHO is also looking into whether the strength and length of immune response depends on the type of infection a person has: without symptoms (‘asymptomatic’), mild or severe. Even people without symptoms seem to develop an immune response. Globally, data from seroprevalence studies suggests that less 10% of those studied have been infected, meaning that the vast majority of the world’s population remains susceptible to this virus. For other coronaviruses – such as the common cold, SARS-CoV-1 and Middle East Respiratory Syndrome (MERS) – immunity declines over time, as is the case with other diseases. While people infected with the SARS-CoV-2 virus develop antibodies and immunity, we do not yet know how long it lasts. Watch this conversation with Dr Mike Ryan and Dr Maria Van Kerkhove for more information on immunity. Large scale physical distancing measures and movement restrictions, often referred to as ‘lockdowns’, can slow COVID‑19 transmission by limiting contact between people. However, these measures can have a profound negative impact on individuals, communities, and societies by bringing social and economic life to a near stop. Such measures disproportionately affect disadvantaged groups, including people in poverty, migrants, internally displaced people and refugees, who most often live in overcrowded and under resourced settings, and depend on daily labour for subsistence. WHO recognizes that at certain points, some countries have had no choice but to issue stay-at-home orders and other measures, to buy time. Governments must make the most of the extra time granted by ‘lockdown’ measures by doing all they can to build their capacities to detect, isolate, test and care for all cases; trace and quarantine all contacts; engage, empower and enable populations to drive the societal response and more. WHO is hopeful that countries will use targeted interventions where and when needed, based on the local situation. WHO TEAM WHO Headquarters (HQ) Related
Emerging data indicate that SARS-CoV-2-specific CD8+ T cells targeting different viral proteins are detectable in up to 70% of convalescent individuals1–5. However, very little information is currently available about the abundance, phenotype, functional capacity and fate of pre-existing and induced SARS-CoV-2-specific CD8+ T cell responses during the natural course of SARS-CoV-2 infection. Here, we define a set of optimal and dominant SARS-CoV-2-specific CD8+ T cell epitopes. We also perform a high-resolution ex vivo analysis of pre-existing and induced SARS-CoV-2-specific CD8+ T cells, applying peptide-loaded major histocompatibility complex class I (pMHCI) tetramer technology. We observe rapid induction, prolonged contraction and emergence of heterogeneous and functionally competent cross-reactive and induced memory CD8+ T cell responses in cross-sectionally analyzed individuals with mild disease following SARS-CoV-2 infection and three individuals longitudinally assessed for their T cells pre- and post-SARS-CoV-2 infection. SARS-CoV-2-specific memory CD8+ T cells exhibited functional characteristics comparable to influenza-specific CD8+ T cells and were detectable in SARS-CoV-2 convalescent individuals who were seronegative for anti-SARS-CoV-2 antibodies targeting spike (S) and nucleoprotein (N). These results define cross-reactive and induced SARS-CoV-2-specific CD8+ T cell responses as potentially important determinants of immune protection in mild SARS-CoV-2 infection. Functionally competent memory CD8+ T cells specific for different viral epitopes are induced by SARS-CoV-2 infection and can be detected in the absence of virus-specific antibodies.
|


 Your new post is loading...
Your new post is loading...