
|
Scooped by Gilbert C FAURE |
https://medicalxpress.com/news/2021-05-years-discovery-immune-dendritic-cells.html
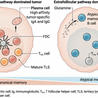
Dendritic cells were discovered in 1868, and at that time were misunderstood and wrongly categorized as members of the nervous system. But immunologists now know there are different types of these cells, even though they all look alike and have roughly the same job as sentinels in the immune system –on patrol 24/7, hunting down infiltrating causes of infection and disease. What separates one group from another, scientists in Germany have just found, is their response to certain signaling molecules and how long they survive in tissues and the blood.
Type 1 dendritic cells tend to undergo regulated cell death after inflammasomes activate. But the investigators found that automatic death wasn't inevitable for type 2 DCs, which did not succumb after inflammasome activation. Type 2 DCs not only survived, but continued their role as a bridge between the innate and adaptive immune systems. The researchers suggest that these cells may be prime targets for approaches to treat inflammatory diseases or to boost the effects of vaccines and adjuvants.
"When conventional type 2 dendritic cells were stimulated with ligands that weakly activated the inflammasome, the DCs did not enter [programmed cell death], but instead secreted interleukin-12 family of cytokines [IL-12] and interleukin-1β [IL-1β]. These cytokines induced prominent T helper type 1 cells and T helper 17 responses," the scientists wrote.


 Your new post is loading...
Your new post is loading...





https://vaporwavepsychedelic.com
https://vaporwavepsychedelic.com/product/buy-2-c-b-drug/
https://vaporwavepsychedelic.com/product/buy-4-aco-dmt/
https://vaporwavepsychedelic.com/product/buy-5-meo-dmt/
https://vaporwavepsychedelic.com/product/ayahuasca-tea/
https://vaporwavepsychedelic.com/product/buy-changa/
https://vaporwavepsychedelic.com/product/dmt-vape-pen/
https://vaporwavepsychedelic.com/product/golden-teacher-mushrooms/
https://vaporwavepsychedelic.com/product/ibogaine-for-sale/
https://vaporwavepsychedelic.com/product/ketamine-drug/
https://vaporwavepsychedelic.com/product/buy-khat-plant-online/
https://vaporwavepsychedelic.com/product/buy-kratom-powder-online/
https://vaporwavepsychedelic.com/product/liberty-caps-mushrooms/
https://vaporwavepsychedelic.com/product/liquid-lsd/
https://vaporwavepsychedelic.com/product/buy-lsd-gel-tabs/
https://vaporwavepsychedelic.com/product/buy-lsd-gummies/
https://vaporwavepsychedelic.com/product/buy-lsd-tabs/
https://vaporwavepsychedelic.com/product/mdma-drug/
https://vaporwavepsychedelic.com/product/peyote-cactus/
https://vaporwavepsychedelic.com/product/buy-pcp-drug/
https://vaporwavepsychedelic.com/product/penis-envy-mushrooms/
https://vaporwavepsychedelic.com/product/buy-dmt/