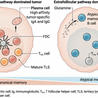
Germinal center (GC) B cells are essential to generating protective antibody responses and are selected through a process of affinity maturation. Kwak et al . now define intrinsic properties of human GC B cells that are critical to antigen affinity discrimination. They identified BCR-containing actin-rich pod-like structures that facilitated formation of highly stable synapses and antigen internalization but only when GC B cells engaged high-affinity antigens. These findings reveal the importance of these structures in setting thresholds for affinity selection and driving GC B cell responses.
Protective antibody responses to vaccination or infection depend on affinity maturation, a process by which high-affinity germinal center (GC) B cells are selected on the basis of their ability to bind, gather, and present antigen to T follicular helper (Tfh) cells. Here, we show that human GC B cells have intrinsically higher-affinity thresholds for both B cell antigen receptor (BCR) signaling and antigen gathering as compared with naïve B cells and that these functions are mediated by distinct cellular structures and pathways that ultimately lead to antigen affinity– and Tfh cell–dependent differentiation to plasma cells. GC B cells bound antigen through highly dynamic, actin- and ezrin-rich pod-like structures that concentrated BCRs. The behavior of these structures was dictated by the intrinsic antigen affinity thresholds of GC B cells. Low-affinity antigens triggered continuous engagement and disengagement of membrane-associated antigens, whereas high-affinity antigens induced stable synapse formation. The pod-like structures also mediated affinity-dependent antigen internalization by unconventional pathways distinct from those of naïve B cells. Thus, intrinsic properties of human GC B cells set thresholds for affinity selection.

|
Scooped by Gilbert C FAURE |
No comment yet.
Sign up to comment


 Your new post is loading...
Your new post is loading...